一時出遊一時爽,一直出遊一直爽!不過,出門玩的時候,若是待在戶外太久,很容易被刺眼陽光曬傷(啊啊啊是破壞死光啊)。這時候為了不要被曬傷,就應該塗防曬乳,對吧?
但是,你知道其實大部分的防曬乳都沒有經過藥品的安全測試嗎?

等等,你說沒有經過測試是什麼意思!?
在美國,他們將防曬乳視為能防止曬傷和皮膚癌的藥,所以由美國食品藥品監督管理局 (U.S. Food and Drug Administration,縮寫為 FDA) 來管理。
從前,在美國賣藥品是不需要任何數據及資料來支持其效力的;但是,在 1962 年,一款緩解孕吐的鎮靜劑在西歐導致成千上萬的嬰兒出現嚴重的先天性缺陷,所以美國 FDA 修法,要求藥品製造商必須科學地證明藥物不僅安全,而且有效。
然而,修法曠日廢時,當終於完成的時候,已經有超過 10 萬種非處方藥在市面上流通,其中便包括了本文的主角──防曬乳。
面對這樣的狀況,美國 FDA 的處理方式是:為它們一個一個列出成分表,如果有公開數據證明這些成分通常是安全有效的,就不用再做進一步檢驗。
正因如此,才會導致現今大部分的防曬乳都跳過了藥品安全測試這一關。

擦在皮膚上的防曬乳,原來可以滲入血液中
但是在今年 (2019) 五月,美國醫學會雜誌 (Journal of the American Medical Association) 刊登了一篇美國 FDA 對防曬乳的隨機臨床試驗結果。(Matta et al., 2019)
他們讓 24 位受試者每天塗四次,持續四天,三天過後抽取血樣並進行分析。
結果,這次的分析確定了市售防曬乳中,有四種化學物質會通過皮膚滲入血液,而且滲入的量讓美國 FDA 認為需要進一步的毒理學測試,以確認是否會導致癌症或其他不良缺陷。
這四種化學物質分別是苯甲酮 (Oxybenzone)、阿伏苯宗 (avobenzone)、奧克立林 (Octocrylene) 還有依莰舒 (ecamsule)。
嘿!防曬乳的研究大概比想像難多了
雖然我們可以嘗試藉由臨床測試來了解藥物對人體帶來的影響,但是,在沒有大規模長期研究的情況下,其實多半無法預測它多年後所產生的後續影響。
因此,單憑缺乏負面證據並不能證明防曬乳是安全的。
另一方面,有動物研究結果發現,防曬乳中吸收紫外線的分子 (UV filter),可能會對生殖、發育還有免疫等等多方面產生不利影響,甚至破壞內分泌系統,而前面提到的苯甲酮 (Oxybenzone) 便是其中一員。
等等,所以防曬乳會讓我們不孕不育嗎?別那麼快下結論。
前美國 FDA 專員、杜克大學醫學院教授羅伯特卡利夫博士 (Dr. Robert Califf) 便解釋道:就算 UV filter 可能會在人體中造成類似風險,但目前並沒有證據證明真是如此。另一 方面,大族群中個體之間的微小差異其實很重要,但我們很難發現這些差異。女性無法懷孕、男性無法生育……這些事情每天都在發生,我們不會想到「噢!一定是防曬乳害的」,當然,也可能真的不是防曬乳害的。
這樣聽起來,防曬乳的研究實在是難以進行啊!
怎麼辦,這樣還要擦防曬乳嗎?

別緊張別緊張,如果因為這樣就不擦防曬乳的話,毒辣的太陽可是會導致曬傷的!而且,美國 FDA 目前也維持原有立場,認為人們該繼續擦防曬,畢竟,防曬乳能夠防止曬傷及皮膚癌是不爭的事實。
美國 FDA 同時也要求製造商們從事進一步研究,且必須在期限內提供額外的數據及資料,否則將可能將他們製造的防曬乳下架。
那麼,有沒有更安心的選擇呢?
不過,其實也不是所有防曬乳都含有上述的化學物質喔!防曬乳根據防曬原理及成份可以分為三種:物理性、化學性還有混合性防曬乳。
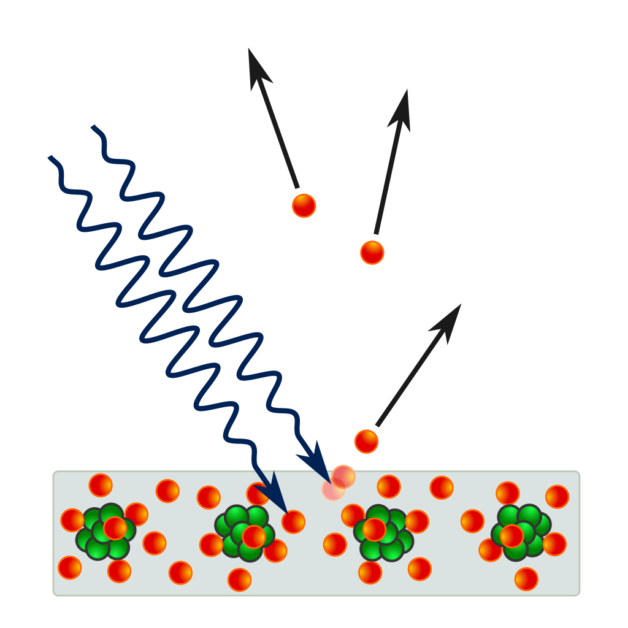
物理性防曬乳的防曬原理,是藉由防曬乳中的二氧化鈦 (TiO2) 跟氧化鋅 (ZnO) 將紫外線反射、折射或是散射掉,藉此達到防曬的作用;而化學性防曬乳則是由分子吸收紫外線後,將其轉換為熱能或其他能量釋放掉,以達到防曬的效果。如果是物理性防曬乳,就沒有那些會滲入皮膚的化學物質了!
辨認防曬乳的方法其實非常簡單,只要看看成份表上面有什麼就好!若是成份只有二氧化鈦 (TiO2) 跟氧化鋅 (ZnO) 的話,就是物理性防曬乳;如果缺少上述兩種成分,而是含有其他化學物質的話,那就是化學性防曬乳。而既有二氧化鈦和氧化鋅,也有其他化學物質的,就是混合性的防曬乳啦!
不過啊,雖然物理性防曬乳並不會有化學物質滲入皮膚的問題,與此同時,它也沒有化學性防曬乳來得清爽,擦起來會比較油膩、厚重,而且比較容易顯白,有較重的粉末感喔!
目前市面上大部分流通的防曬乳其實都是混合型防曬乳,各位如果有特殊偏好,在購買防曬乳時還請注意標示,別買錯囉!

最後,雖然一直出遊一直爽,但遇到曬傷可就不太爽啦!所以夏日出門,還是記得要擦防曬喔!否則等到曬傷脫皮時再來懊悔就沒用啦!
參考資料
- The New York Times Magazine : When You Wear Sunscreen, You’re Taking Part in a Safety Study
- The New York Times Magazine : How Safe Is Sunscreen?
- U.S Food & Drug Administratuion : Kefauver-Harris Amendments Revolutionized Drug Development
- 美的好朋友:物理性防曬和化學性防曬到底哪個好?關鍵5點讓你秒懂!防曬全攻略
- CASE 報科學:【生活化學】防曬乳科學:是保護還是另一種傷害?
- Matta, M. K., Zusterzeel, R., Pilli, N. R., Patel, V., Volpe, D. A., Florian, J., … & Kemp, S. (2019). Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. Jama, 321(21), 2082-2091.