疫情期間學烹飪,再拍照上傳社群網站,是凡夫俗子的成果發表;將煮義大利麵的心得筆記,發表在《流體物理學》(Physics of Fluids)期刊上,[1]則是科學家的華麗炫技。
煮麵的動機
美國伊利諾大學Sameh Tawfick副教授的實驗室,專攻靈活可變形的纖維和有彈性的結構,所產生的「流體結構交互作用」(fluid structure interaction)。「過去幾年老是開玩笑,說義大利麵的黏著力與我們的研究息息相關」,他說團隊發覺分析麵條力學質地的轉變,「可以體現黏著力、力學質地和烹煮熟度的關聯。」[2]
以上研究動機有聽沒懂,無所謂。煮麵要緊。
煮麵的方法

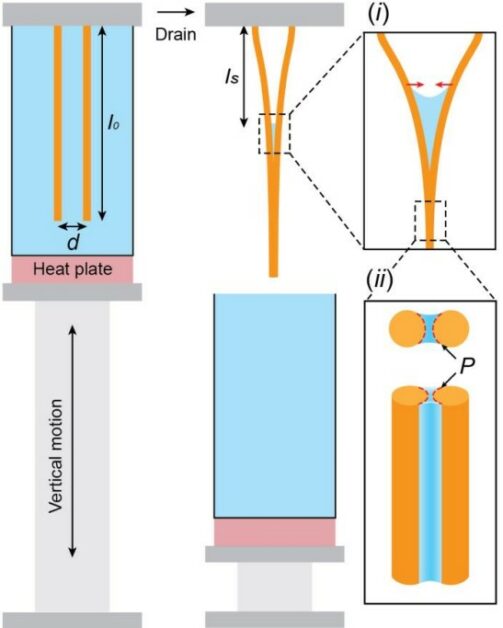
傳統義大利麵講求的口感,叫做「al dente」,意思是「煮到內硬外軟」,恰到好處。從物理的角度來看,水份由麵條表面,逐漸擴散進入內部,所以首先軟化的當然是最外層。吸水的過程中,麵條體積會隨之膨脹。煮愈久,效果愈明顯。下圖是研究團隊在觀察義大利麵「吸濕膨脹」(hygroscopic swelling)時,進行的量化紀錄。[3]
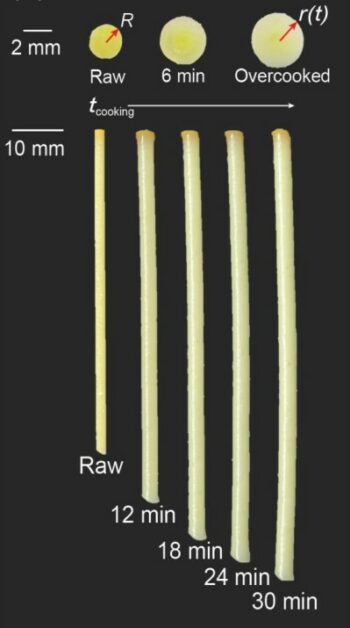
所以,到底要怎麼做,才能擁有al dente的口感?
研究團隊發現義大利麵條達到 al dente 前,其周長與長度分別的膨脹率相比,所得的比率是3.5比1。一旦超過了,就會軟爛。[2]此外,由於麵條煮愈久,離水時彼此相黏的部份就愈長。研究團隊認為,專業廚師也可以測量相黏長度,來推論起鍋時間,以後再將成熟的技術,推廣至普通民家…[3](原文口氣意外地認真。)總之,要知道煮好沒,不是用嘴試吃,也別拿錶計時,科學家的建議竟是用尺測量!
煮麵的鹽和光
明明義大利麵條的包裝上,都有建議的烹煮時間。為什麼科學家不直接告訴大家,煮多久能起鍋?原來如果照正常的煮法,在水中加食鹽,麵條的化學和力學特性都會起變化。比起用蒸餾水,鹽水不僅有助麵條膨脹,而且會增添嚼勁。[3]此外,Sameh Tawfick 副教授解釋,滾水中的鹽量,會改變達到 al dente 所需的時間。有鑑於此,他未來要探討食鹽,在義大利麵膨脹時所扮演的角色。[2]同時,這個研究正如一道照亮前程的光,或許會引領其他人,也來嘗試用簡單的方式,研究軟物質的特性。
參考資料:
- Hwang J., Ha J., Siu R., Kim Y. S., and Tawfick S. (2022) ‘Swelling, Softening, and Elastocapillary Adhesion of Cooked Pasta’, Physics of Fluids, 34 (042105)
- Physics Models Better Define What Makes Pasta Al Dente (Physics of Fluids, 2022)
- Hwang J., Ha J., Siu R., Kim Y. S., and Tawfick S. (2022) ‘Swelling, Softening and Elastocapillary Adhesion of Cooked Pasta’, arXiv
- Hot Plate Use and Safety in Laboratory (University of Wisconsin-Madison, 2013)
- Spaghetti and Meatballs (Gourmet Traveller, 2018)
- 第11章 有趣的界面現象(國立成功大學化學工程學系)
- Chemical Science Lesson Plan: Hydrogen Bonding and Surface Tension (University of Illinois, 2010)
- Enjoy better-cooked pasta with…physics and a ruler? (University of Illinois, 2022)
-200x200.jpg)